Amphibian Metamorphosis Assay (AMA)
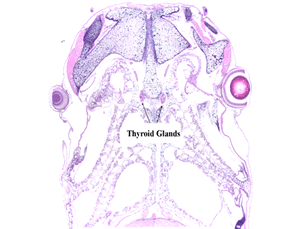
The EDSP Tier 1 EPA 890.1100/OECD 231 AMA is a 21-day test using prometamorphic Xenopus larvae to detect EDCs that alter the hypothalmo-pituitary-thyroid (HPT) axis. The test is initiated using NF stage 51 X. laevis larvae. At the conclusion of exposure, measurements of development stage, growth, and thyroid gland histopathology are performed.

Larval Amphibian Growth and Development Assay (LAGDA)
The EDSP Tier 2 EPA 890.2300/OECD 241 is a 10 weeks post-metamorphosis (wpm) test using Xenopus to detect EDCs that alter both the hypothalmo-pituatary-thyroid (HPT) and gonad (HPG) axes. The test is initiated with NF stage 8-10 embryos. Intermediate endpoints at metamorphic climax (NF stage 62) are collected including time to metamorphosis, growth, and thyroid gland histopathology are performed. At 10 wpm, measurements of survival, growth, phenotypic/genotypic sex, and gonad histopathology are measured.
Full and Partial Lifecycle Amphibian Toxicity Assay
We have developed a standard protocol of evaluating reproductive toxicity in Xenopus. The current protocol entails a 30-45 day exposure or dosing period following super-ovulation, followed by a full reproductive work-up which includes mating, embryo counts, fertilization and necrosis rates, viability assessment, and short-term (4-d) teratological examination. An evaluation of ovary pathology, oocyte count, stage, and viability, testis pathology, sperm count, and dysmorphology assessment is also performed as warranted. Crossover experiments in which exposed females are mated with unexposed males and are also commonly performed with the assay. Dosing/exposure may be accomplished by parenteral routes, oral (intubation or feed), or by ambient exposure in the culture water or natural site waters/sediments. These protocols have also been adapted for use with native species, including frogs and toads (Lithobathes sp. and Bufo sp.)

EFED Acute Oral Gavage Method – Pesticide Toxicity in Terrestrial Amphibians
An acute oral toxicity test with a terrestrial-phase amphibian is necessary to remove the uncertainty within the field of agrochemical risk assessment, where for risk assessments conducted under FIFRA, endpoints for avian species are typically used as a surrogate in the absence of toxicity data for terrestrial-phase amphibians. We use the bullfrog (Lithobates catesbeianus) most commonly in these studies as it is a representative of the family Ranidae) and historically this species has been used as an amphibian test model species. However, we have used other species of frogs and toads depending on the circumstances. Anesthetization prior to dose administration to successfully reduces or eliminates the potential for regurgitation which is often problematic with amphibians. Juvenile bullfrogs are then administered a single dose of test article via oral gavage of a single gelatin capsule or neat liquid, returned to their respective aquaria, and monitored for survival for 14 days.
Fish Short-Term Reproduction Assay (FSTRA)
The EDSP Tier 1 EPA 890.1350/OECD 229 FSTRA is a 21-day test using reproductively mature fathead minnows to detect EDCs that alter the hypothalmo-pituitary-gonad (HPG) axis. The test is initiated using reproductively viable minnows, but can be adapted to use medaka or zebrafish. At the conclusion of exposure, measurements of survival, reproductive fecundity (egg production, fertilization, and hatching success), gonado-somatic indices (GSI), vitellogenin levels, and gonad histopathology histopathology are performed.


Medaka Extended One-Generation Reproductive Toxicity Assay (MEOGRT)
The EDSP Tier 2 EPA 890.2200 MEOGRT is a modified lifecycle assay using mature medaka to detect EDCs that alter the hypothalmo-pituitary-gonad (HPG) axis. The test is initiated using reproductively viable medaka. At the conclusion of each generation (F0 and F1), measurements of survival, reproductive fecundity (egg production, fertilization, and hatching success), gonado-somatic indices (GSI), and gonad histopathology histopathology are performed. Intermediate endpoints are collected with F1 sub-adult fish including survival, growth, liver vitellogenin expression, phenotypic/genetic sex, and gonad/thyroid histopathology.